In cold weather, the discharge voltage and capacity of lithium batteries are reduced to varying degrees. Research has found that when lithium batteries are discharged at -20°C, they only have about 30% of their rated capacity, which means that lithium battery capacity is depleted much faster than in summer.
Why are lithium batteries "afraid of cold" just like people? What chemical changes will occur in lithium batteries in low-temperature environments? That’s what we’re going to talk about today.
The impact of cold weather on lithium batteries
Cold weather is harmful to lithium batteries.In chilly conditions, specifically below 0°C, lithium batteries are susceptible to the adverse effects of low temperatures. This triggers lithium precipitation within the battery, leading to the thickening of the Solid Electrolyte Interphase (SEI) film, an increase in electrolyte concentration, and the simultaneous formation of lithium dendrites. Consequently, these chemical reactions contribute to a decrease in capacity, making lithium batteries more prone to depletion in cold weather.
1. Low-temperature charging can lead to lithium precipitation
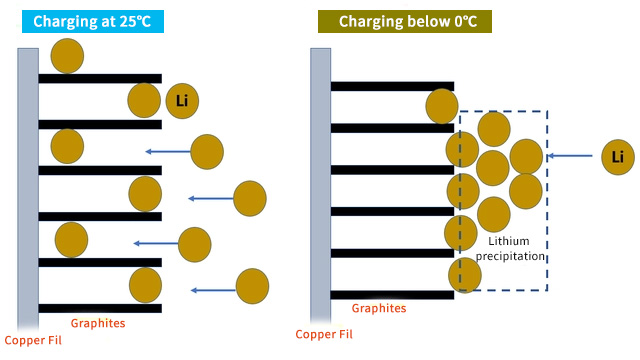
When lithium batteries are charged at low temperatures, the electrochemical reaction and solid diffusion slow down, and the material lattice shrinks. The lithium ions from the positive electrode do not have time to reach the graphite layer, and nd turn into metallic lithium on the surface of the negative electrode. This is lithium precipitation. Lithium precipitation can easily form lithium dendrites, and larger dendrites can even pierce the separator and cause damage to the battery.
2. SEI film becomes thicker
The SEI membrane is mainly composed of lithium alkyl ester, lithium carbonate, etc. It has a multi-layer structure, and the end close to the electrolyte is denser. The membrane acts as an intermediate phase between the electrode and the electrolyte, has the properties of a solid electrolyte, and only allows lithium Ions to pass freely and are insulated from electrons.
At low temperatures, the resistance of the SEI film increases, the negative electrode potential shifts to a low potential, and lithium ions are more likely to be precipitated, causing the organic electrolyte to decompose to form a new SEI film, which will lead to fewer active lithium ions in the battery. We all know that the capacity of lithium batteries is determined by the number of lithium ions.
3. The viscosity of the electrode solution increases
In low-temperature environments, the viscosity of the electrolyte increases and even partially solidifies, causing the conductivity of lithium-ion batteries to decrease.
In addition, under low-temperature conditions, the ohmic internal resistance of lithium-ion batteries will increase.
Do lithium batteries work in cold weather?
We have discussed the impact of low temperature on lithium batteries above, but these factors are not enough to "kill" the battery. The confrontation between lithium batteries and cold weather is ultimately reflected in the design and preparation process.

In the market, some manufacturers have introduced "cold-temperature" lithium batteries, claiming that they can charge normally at -20°C and discharge at -50°C. These cold-temperature lithium batteries typically employ special materials and structural designs to enhance their performance and safety in low-temperature environments. In addition, some electric vehicle manufacturers and mobile phone battery manufacturers have also begun to develop low-temperature battery technology suitable for cold environments to meet market demand.
Guidelines for using lithium batteries in cold weather
If you aim to use your lithium battery devices normally in cold winter environments while minimizing potential damage to the batteries, consider charging them in an indoor area with temperatures above 0°C. Additionally, adopting a strategy of shallow charging and discharging, along with utilizing low current for charging, can be beneficial.
1. Charging in an environment higher than 0℃
When the temperature is below 0°C, the charging and discharging efficiency of lithium batteries is greatly reduced. At extremely low temperatures, such as -20°C, ordinary lithium batteries will trigger low-temperature protection and enter a dormant state. At this time, the lithium battery or device should be moved indoors and recharged when the temperature is slightly normal, but please be careful to keep away from flammable items.
2. Shallow discharge and shallow charging
In winter, when the battery power is lower than 30%, we need to charge it in time. It is best to charge it to about 80%.
The activity of lithium batteries decreases in low-temperature environments. If they are deeply charged and discharged, it is easy to cause over-discharge and over-charge. Also, be careful not to leave the charger plugged in to charge your device or vehicle.
3. Use small current to charge
Research has found that charging the battery at a high rate when the battery is low, and then charging with a small current as the battery increases, can avoid lithium precipitation. In low-temperature environments, charging should be performed with a smaller current. After charging, let the lithium-ion battery stand for a certain period of time, and the precipitated metallic lithium will be re-embedded in the graphite crystal, reducing the loss of active lithium.
Storing lithium batteries in low-temperature environments
The above content is the use of lithium batteries in low-temperature environments, so you may have questions. If you just store lithium batteries without using them, will the battery capacity be lost?

If the lithium battery is not used, will the low-temperature cause damage to the battery?
Before answering, we need to know the aging mechanism of lithium-ion batteries: calendar aging and cycle aging. Cycle aging is a dynamic charge and discharge process, and calendar aging is aging during non-use storage.
Calendar aging is affected by temperature and SOC. Under high temperatures and high SOC, the stability of the electrode/electrolyte interface decreases, and side reactions increase - the positive electrode metal ions dissolve, oxygen evolves, the electrolyte decomposes, and the SEI film on the negative electrode surface thickens.
Therefore, low temperatures mitigate the calendar aging process to a certain extent. It does not cause irreversible loss of lithium-ion batteries during storage.
How to store
If the battery is not used for a long time, remember to charge it to 50%-80% capacity, remove it from the device, and charge it regularly every month. If it cannot be disassembled, just charge it regularly. Note: Batteries must be stored in a dry environment.
 sales@batterydeji.com
sales@batterydeji.com




