In everyday life, we might confuse the terms positive and negative electrodes with anode and cathode, but in fact, their definitions differ. Physics defines the positive and negative electrodes, while electrochemistry defines the anode and cathode.
What are the positive and negative electrodes of a battery?
The positive and negative electrodes are defined by physics. In a battery, the positive electrode (Positive) refers to the electrode with relatively higher voltage, and the negative electrode (Negative) has relatively lower voltage. For example, in an iPhone battery, the voltage of lithium cobalt oxide (LiCoO2) is always higher than that of graphite, thus LiCoO2 is the positive electrode material, while Graphite is the negative electrode material.
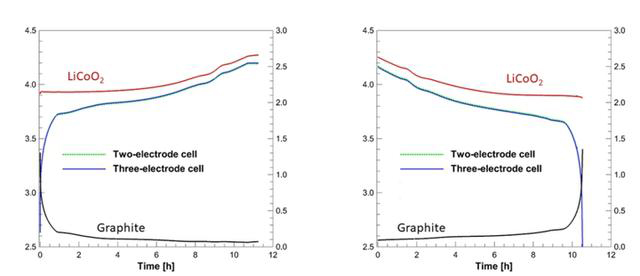
It's important to note that the positions of the positive and negative electrodes are not fixed. During discharge, electrons flow out of the battery's negative electrode, pass through the external circuit, and enter the positive electrode. During charging, electrons flow from the external power source into the negative electrode and out from the positive electrode, at which point the roles of the battery's positive and negative electrodes are reversed.
What are the anode and cathode?
In electrochemistry, the definitions of anode and cathode relate to the direction of the current. The anode is the electrode where oxidation occurs, losing electrons, while the cathode is the electrode where reduction occurs, gaining electrons. During battery charging, the roles of the anode and cathode are reversed; that is, the anode during discharge becomes the cathode during charging, and vice versa.
However, in everyday life, we are also accustomed to considering the negative electrode as the anode and the positive electrode as the cathode. Everyone generally accepts this terminology as long as we know which specific electrode is being described.
What materials are used for the positive and negative electrodes of a battery?
The most popular batteries on the market today are lithium-ion batteries, nickel-metal hydride batteries, and lead-acid batteries, and they use different materials for their positive and negative electrodes.
Lithium-Ion Batteries
Positive Electrode Materials: Typically use lithium-containing metal oxides such as lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), lithium manganese oxide (LiMn2O4), and nickel manganese cobalt oxide (LiNiMnCoO2). These materials receive lithium ions released from the negative electrode during battery discharge.
Negative Electrode Materials: Commonly graphite, as well as advanced materials such as silicon or tin-based alloys. These materials release lithium ions during battery discharge.
Lead-Acid Batteries
Positive Electrode Materials: Lead dioxide (PbO2), which transforms into lead sulfate (PbSO4) during discharge.
Negative Electrode Materials: Pure lead (Pb), which also transforms into lead sulfate (PbSO4) during discharge.
Nickel-Metal Hydride Batteries
Positive Electrode Materials: Nickel hydroxide (Ni(OH)2), which effectively stores and releases hydrogen ions during discharge.
Negative Electrode Materials: Metal hydrides (such as rare-earth metal hydrides or zinc titanate), which release electrons during discharge.
How to Identify the Positive and Negative Electrodes of a Battery?
Identifying the positive and negative electrodes on a battery is crucial for correctly connecting external circuits.
1. Check the Battery Markings: Some batteries, such as cylindrical batteries and button cells, are marked with a "+" sign for the positive electrode and a "-" sign for the negative electrode. This is the most common and simplest method.
2. Observe the Length of the Battery: In some batteries, the positive and negative electrodes differ in length, with the positive electrode being slightly longer. In such cases, the length of the battery can be used to distinguish between the positive and negative electrodes.
3. Color Coding: Some batteries use different colors to distinguish between the positive and negative electrodes, such as red for the positive and black for the negative. In such cases, the color of the battery can be used to distinguish between the positive and negative electrodes.
4. Using a Multimeter for Testing: If the above methods do not determine the positive and negative electrodes of the battery, a multimeter can be used for testing. Connect the positive and negative leads of the multimeter to the two ends of the battery. If the multimeter displays a positive voltage, then the end connected to the multimeter's positive lead is the positive electrode of the battery, and vice versa.
In addition to these methods, there is also a special type of battery called a "non-polarized battery," which does not have a clear positive or negative electrode distinction and can be connected in any direction without damaging the circuit. Non-polarized batteries are typically used in small electronic devices, such as watches and remote controls.
 sales@batterydeji.com
sales@batterydeji.com




