Before understanding the working principle of mobile phone lithium battery, we first need to have a general understanding of its composition.
Composition of mobile phone lithium battery
Mobile phone batteries are mainly composed of 6 parts: positive electrode, separator, negative electrode, organic electrolyte, battery protection plate, and battery casing.
Positive electrode
The active material of mobile phone batteries is generally lithium manganate or lithium cobalt oxide, lithium nickel cobalt manganate material. Electric bicycles generally use lithium nickel cobalt manganate (commonly known as ternary) or ternary + a small amount of lithium manganate. Pure lithium manganate and lithium iron phosphate are gradually fading out due to their large size, poor performance or high cost. The conductive electrode fluid uses electrolytic aluminum foil with a thickness of 10-20 microns.
Separator
A specially formed polymer film. The film has a microporous structure that allows lithium ions to pass through freely, but electrons cannot pass through.
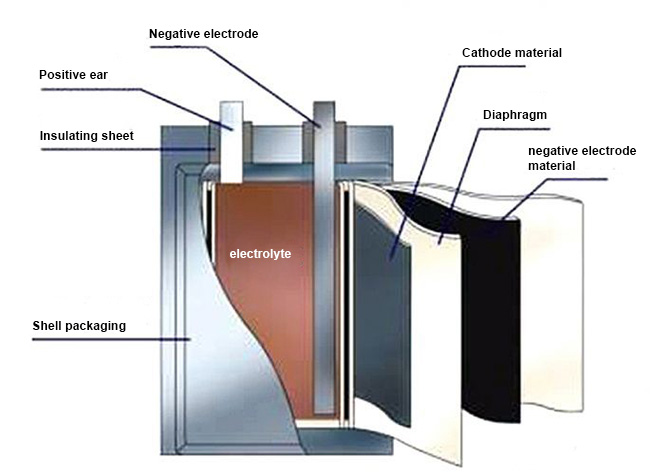
Negative electrode
The active material is graphite or carbon with a graphite structure, and the conductive current collector uses electrolytic copper foil with a thickness of 7-15 microns.
Organic electrolyte
A carbonate solvent in which lithium hexafluorophosphate is dissolved. Lithium-ion polymer batteries use gel electrolytes.
Battery shell
Divided into steel shell (square type is rarely used), aluminum shell, nickel-plated iron shell (used for cylindrical batteries), aluminum-plastic film (soft packaging), etc., as well as the battery cap, which is also the positive part of the battery. Negative terminal.
Battery protection board

As the name suggests, the battery protection board is an integrated circuit board that protects rechargeable batteries (generally lithium batteries). The reason why lithium batteries (rechargeable types) need protection is that the material of the lithium battery itself determines that it cannot be overcharged, over-discharged, overcurrent, short-circuited, or charged and discharged at ultra-high temperatures. Therefore, lithium batteries always have a protective plate and a current fuse appears.
Working principle of mobile phone lithium battery
The following will introduce the working principle of lithium battery from three parts: the charging process, discharging process, and battery protection board:
(1) When the lithium battery is charging
The positive electrode of the battery is generated by lithium ions. The generated lithium ions "jump" into the electrolyte from the positive electrode, "crawl" through the small winding holes in the separator through the electrolyte, and move to the negative electrode. The electrons from the negative electrode are bonded together.
●The reaction that occurs on the positive electrode is: LiCoO2==charge==Li1-xCoO2+Xli++Xe (electrons)
●The reaction on the negative electrode is 6C+XLi++Xe======LixC6. During the charging process, Li+ comes out of the positive electrode LiCoO2, enters the electrolyte, moves toward the negative electrode under the action of the external electric field attached to the charger, and sequentially enters the negative electrode composed of graphite or coke C, forming LiC compounds at the negative electrode.
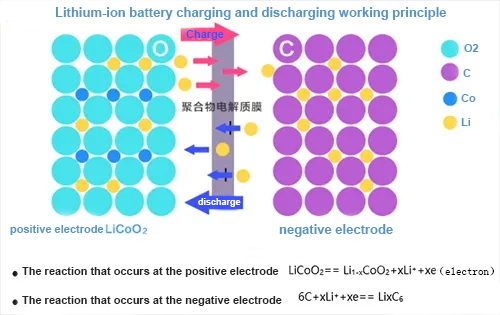
(2) When the lithium battery is discharging
During discharge, both electrons and Li+ move at the same time, in the same direction but in different paths. The electrons run from the negative electrode to the positive electrode through the external circuit; the lithium ions Li+ "jump" into the electrolyte from the negative electrode and "crawl" through the twists and turns on the separator. The small hole "swims" to reach the positive pole and is combined with the electrons that have already run over. What we usually call battery capacity refers to the discharge capacity.
(3) Battery protection board
The picture below shows the battery panel protection circuit. PTC: Positive temperature coefficient thermistor; NTC: Negative temperature coefficient thermistor. When the ambient temperature rises, its resistance decreases. The electrical equipment or charging equipment can respond in time to control internal interruptions and stop charging and discharging; U1 is the Circuit protection chip, and U2 is two reverse-connected MOSFET switches. Under normal conditions, both CO and DO of the battery board U1 output high voltage, both MOSFETs are turned on, and the battery can be charged and discharged freely.
Overcharge protection:
When U1 detects that the battery voltage reaches the overcharge protection threshold, the CO pin outputs a low level, MOS switch 2 turns from on to off, the charging circuit is turned off, and the charger can no longer charge the battery, thus realizing Overcharge protection.
Over-discharge protection:
Battery discharge process, when U1 detects that the battery voltage is lower than the overcharge protection threshold, the DO pin changes from high level to low level, and the MOS tube switch 1 is turned off so that the battery cannot be discharged; over-discharge In the protection state, the battery voltage cannot be reduced any more, which requires the current of the protection circuit to be extremely small and the control circuit to enter low power consumption. Overcurrent protection: Under normal circumstances, the battery discharges the load, and the current passes through two series-connected MOS tube switches. The VM pin detects that the voltage drop of the two MOS tubes is U. If the load causes U to be abnormal for some reason, the loop current increases. When U is greater than a certain value, the DO pin changes from high voltage to low voltage, and the MOS tube switch 1 is closed, so that the discharge loop current is zero and reaches over Current protection function.
 sales@batterydeji.com
sales@batterydeji.com




