Lithium batteries are at the heart of modern portable electronics, electric vehicles, and energy storage systems. One of the key components that enable these batteries to function effectively is the electrolyte. Acting as the "blood" of the battery, the electrolyte facilitates the movement of lithium ions between the positive and negative electrodes, enabling the charging and discharging process. In this article, we will explore the composition of lithium battery electrolytes, their vital roles, and how they differ from electrolytes used in other types of batteries, such as nickel-metal hydride and lithium-polymer batteries
What is a Lithium Battery Electrolyte?
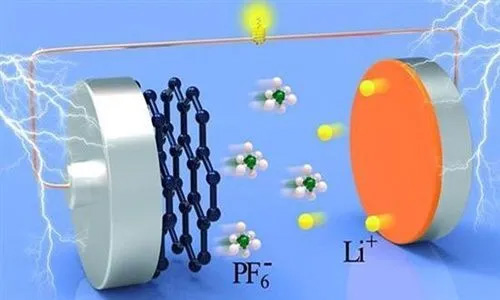
A lithium battery electrolyte is a conductive medium located between the battery's positive and negative electrodes. During charging and discharging, the electrolyte acts as a conductor for lithium ions, responsible for transmitting lithium ions between the positive and negative electrodes. Simply put, the lithium battery electrolyte is like the "blood" of the battery. It builds a bridge between the positive and negative electrodes, allowing lithium ions to "swim" smoothly across, enabling the battery's charge and discharge processes.

Composition of Lithium Battery Electrolyte
The electrolyte is mainly composed of three parts:
-
Solvent: Typically, carbonate compounds such as ethylene carbonate (EC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC) are used. These solvents provide good conductivity and chemical stability. They allow the lithium salt to dissolve, creating a comfortable "swimming environment" for lithium ions.
-
Lithium Salt: This is the "salt" in the electrolyte, with lithium hexafluorophosphate (LiPF6) being the most common. Its role is to provide a large number of lithium ions, allowing these ions to shuttle back and forth between the positive and negative electrodes, completing the transfer of charge.
-
Additives: To enhance battery performance and safety, some additives are included in the electrolyte. These additives can promote the formation of a solid electrolyte interface (SEI) film, which acts like a protective barrier at the edge of the pool, protecting the lithium ions while swimming and reducing unnecessary chemical reactions, thus extending the battery's life.
Functions of Lithium Battery Electrolyte
The electrolyte in a lithium battery has two key functions:
-
Conductivity: The electrolyte serves as an ionic conductor within the battery. During charging, lithium ions are extracted from the positive electrode material, move through the electrolyte to the negative electrode, and embed there. Conversely, during discharging, lithium ions are extracted from the negative electrode material, move through the electrolyte to the positive electrode, and embed there. This process is like a back-and-forth journey of lithium ions on the electrolyte's "highway," allowing the battery to store and release electrical energy.
-
Stability: The electrolyte helps maintain the stability of the chemical reactions inside the battery, preventing overheating, short circuits, or other potential hazards. For example, the electrolyte assists in forming a solid electrolyte interface (SEI) film that covers the surface of the negative electrode, preventing the electrolyte from directly reacting with the metal lithium, thus reducing side reactions and enhancing the battery's cycle life and safety. Additionally, the composition and properties of the electrolyte can affect the battery's thermal stability, avoiding dangerous situations under high temperatures or overcharging conditions.
Therefore, the electrolyte is not only a medium for lithium ion transmission but also a key factor in ensuring the stability and safety of the battery's internal environment. It plays an irreplaceable role in the battery, being fundamental to the battery's normal operation and high performance.
Differences Between Electrolytes in Nickel-Metal Hydride Batteries and Lithium Batteries
-
Electrolyte Composition
- Lithium Battery Electrolyte: Typically composed of organic solvents, lithium salts (such as LiPF6, LiBF4), and some additives. The lithium salt is the star here, dissolving in the organic solvent, releasing lithium ions, and completing the charge and discharge mission by shuttling between the battery's positive and negative electrodes.
- Nickel-Metal Hydride Battery Electrolyte: Usually composed of potassium hydroxide, lithium hydroxide, and additives. The main characters here are hydrogen ions and hydroxide ions, continuously dissolving and precipitating during the battery's charge and discharge process. Unlike lithium batteries, the ions in the nickel-metal hydride battery electrolyte are larger, move more slowly, and result in a slower discharge rate.
-
Operating Temperature and Stability
- Lithium Battery Electrolyte: Has higher conductivity and adaptability to temperature changes, capable of working in a wide temperature range, making it suitable for applications in mobile devices and electric vehicles. However, the chemical nature of lithium battery electrolyte is more active, posing risks of explosion and fire, requiring strict control of temperature and current during use.
- Nickel-Metal Hydride Battery Electrolyte: Its chemical nature is relatively stable, making it safer and less prone to explosion and fire. However, it is sensitive to environmental humidity, where excess moisture can affect the battery's lifespan and performance. Therefore, it needs to be kept appropriately dry to avoid hydrogen gas generation, reduced battery life, and decreased performance.
Differences Between Electrolytes in Lithium-Ion and Lithium-Polymer Batteries
Although the basic components of electrolytes in lithium-ion and lithium-polymer batteries are similar, both based on organic solvents and lithium salts, their physical forms and packaging technologies differ. Traditional lithium-ion battery electrolytes are liquid, while lithium-polymer batteries have the electrolyte fixed in a polymer matrix, forming a gel-like semi-solid electrolyte. Lithium-ion batteries typically use steel or aluminum casing, while lithium-polymer batteries employ thinner soft-pack casing, allowing them to be designed as thinner, lighter, and more flexible batteries, suitable for applications where space is limited or custom shapes are needed.
 sales@batterydeji.com
sales@batterydeji.com




