1. Introduction of lithium ion battery:
Lithium ion battery is a kind of secondary battery (rechargeable battery), which mainly relies on the movement of lithium ions between the positive electrode and the negative electrode to work. During the charging and discharging process, Li+ intercalates and deintercalates back and forth between the two electrodes: during charging, Li+ deintercalates from the positive electrode and inserts into the negative electrode through the electrolyte, and the negative electrode is in a lithium-rich state; the opposite is true during discharge.
Lithium batteries are divided into lithium batteries and lithium ion batteries. Cell phones and laptops use lithium-ion batteries, which are commonly referred to as lithium batteries. Batteries generally use materials containing lithium as electrodes, which are representative of modern high-performance batteries. The real lithium battery is rarely used in daily electronic products because of its high risk.
Lithium-ion batteries were first successfully developed by Sony Corporation of Japan in 1990. It embeds lithium ions into carbon (petroleum coke and graphite) to form a negative electrode (traditional lithium batteries use lithium or lithium alloy as the negative electrode). LixCoO2 is commonly used as the cathode material, and LixNiO2 and LixMnO4 are also used. The electrolyte uses LiPF6 + diethylene carbonate (EC) + dimethyl carbonate (DMC).
Petroleum coke and graphite are non-toxic as negative electrode materials, and the resources are sufficient. Lithium ions are embedded in carbon, which overcomes the high activity of lithium and solves the safety problems of traditional lithium batteries. The positive electrode LixCoO2 can reach the charging and discharging performance and life. The higher level reduces the cost, and in short, the overall performance of the lithium-ion battery is improved. It is expected that lithium-ion batteries will occupy a large market in the 21st century.
The reaction formula for charging and discharging lithium ion secondary batteries is LiCoO2+C=Li1-xCoO2+LixC
2. Lithium-ion battery components
Steel shell/aluminum shell/cylindrical/soft packaging series:
(1) Positive electrode-the active material is generally lithium manganese oxide or lithium cobalt oxide, and lithium nickel cobalt manganese oxide materials. Electric bicycles generally use lithium nickel cobalt manganese oxide (commonly known as ternary) or ternary + a small amount of lithium manganate, pure Lithium manganese oxide and lithium iron phosphate are gradually faded out due to their large size, poor performance or high cost. The conductive current collector uses electrolytic aluminum foil with a thickness of 10-20 microns.
(2) Diaphragm-a specially formed polymer film with a microporous structure that allows lithium ions to pass freely, but electrons cannot pass.
(3) Negative electrode-the active material is graphite, or carbon with a similar graphite structure, and the conductive current collector uses electrolytic copper foil with a thickness of 7-15 microns.
(4) Organic electrolyte—carbonic acid ester solvent with lithium hexafluorophosphate dissolved, while gel electrolyte is used for polymer.
(5) Battery case-divided into steel case (square type is rarely used), aluminum case, nickel-plated iron case (used for cylindrical batteries), aluminum plastic film (soft packaging), etc., as well as the battery cap, which is also the positive of the battery. Negative terminal
3. Main types of lithium-ion batteries
According to the different electrolyte materials used in lithium-ion batteries, lithium-ion batteries are divided into liquid lithium-ion batteries (Liquified Lithium-Ion Battery, referred to as LIB) and Polymer Lithium-Ion batteries (abbreviated as PLB).
Lithium ion battery (Li-ion)
Rechargeable lithium-ion battery is the most widely used battery in modern digital products such as mobile phones and notebook computers, but it is more "squeaky" and cannot be overcharged or overdischarged during use (it will damage the battery or make it scrap). Therefore, there are protective components or protective circuits on the battery to prevent expensive battery damage. Lithium-ion battery charging requirements are very high. To ensure that the termination voltage accuracy is within ±1%, major semiconductor device manufacturers have developed a variety of lithium-ion battery charging ICs to ensure safe, reliable, and fast charging.
Mobile phones basically use lithium-ion batteries. Proper use of lithium-ion batteries is very important to extend battery life. It can be made according to the requirements of different electronic products
Flat rectangular, cylindrical, rectangular and button type, and there are battery packs composed of several batteries connected in series and parallel. The rated voltage of the lithium ion battery, because of the change of the material It is generally 3.7V, and 3.2V for lithium iron phosphate cathode. The final charging voltage when fully charged is generally 4.2V, and lithium iron phosphate 3.65V. Lithium-ion battery termination discharge the voltage is 2.75V~3.0V (the battery factory gives the operating voltage range or the termination discharge voltage, the parameters are slightly different, generally 3.0V, and phosphorus iron is 2.5V). Less than 2.5V (lithium iron phosphate 2.0V) continues to discharge is called overdischarge, overdischarge will damage the battery.
Lithium-ion batteries with lithium cobalt oxide type materials as the positive electrode are not suitable for high-current discharge. Excessive current discharge will reduce the discharge time (the internal temperature will be higher and the energy will be lost).However, the lithium iron phosphate cathode material lithium battery can be used at 20C or more (C is the capacity of the battery, such as C=800mAh, and the charging rate of 1C is the charging current.For charging and discharging with a large current of 800mA, it is especially suitable for electric vehicles. Therefore, the battery production factory gives the maximum discharge current, which should be less than the maximum discharge current during use.Lithium-ion batteries have certain requirements for temperature. The factory provides the charging temperature range, discharging temperature range and storage temperature range. Overvoltage charging will cause permanent damage to the lithium-ion battery.Bad. The charging current of lithium-ion batteries should be based on the recommendations of the battery manufacturer, and a current-limiting circuit should be required to avoid overcurrent (overheating). The commonly used charging rate is 0.25C~1C. It is often necessary to detect the battery temperature during high-current charging to prevent overheating from damaging the battery or causing an explosion.
Lithium-ion battery charging is divided into two stages: first constant current charging, and changing to constant voltage charging when it is close to the termination voltage. For example, a battery with a capacity of 800mAh, whose termination voltage is 4.2V. The battery is charged with a constant current of 800mA (charging rate of 1C). At the beginning, the battery voltage is boosted with a larger slope. When the battery voltage is close to 4.2V, it is changed to 4.2V constant voltage charging., The current gradually drops, and the voltage changes little. When the charging current drops to 1/10-50C (various factory settings, it does not affect the use), it is considered to be nearly full and the charging can be terminated (with When the charger reaches 1/10C, the timer will start, and the charging will end after a certain period of time).
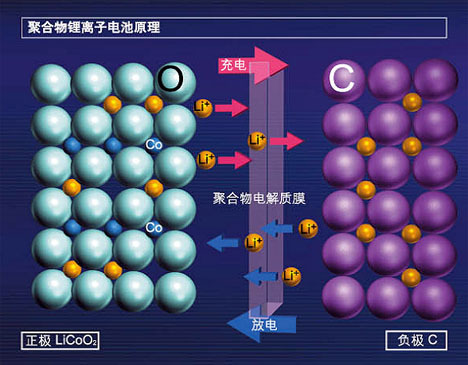
4. The working principle of lithium ion battery
Lithium-ion batteries use carbon materials as the negative electrode and lithium-containing compounds as the positive electrode. There is no metal lithium, only lithium ions, which is a lithium-ion battery. Lithium-ion battery is refers to the general term for batteries with lithium ion intercalation compound as the cathode material. The charging and discharging process of lithium ion batteries is the process of intercalation and deintercalation of lithium ions. In the intercalation and desorption of lithium ions In the process of intercalation, it is accompanied by the insertion and deintercalation of electrons equivalent to lithium ions (usually the positive electrode is represented by insertion or deintercalation, and the negative electrode is represented by insertion or deintercalation). Charging and discharging During the electrical process, lithium ions are intercalated/deintercalated and intercalated/deintercalated back and forth between the positive and negative electrodes, which is vividly called the "rocking chair battery".
When the battery is charged, lithium ions are generated on the positive electrode of the battery, and the generated lithium ions move to the negative electrode through the electrolyte. The carbon as the negative electrode has a layered structure, which has there are many micropores, and the lithium ions reaching the negative electrode are inserted into the micropores of the carbon layer. The more lithium ions are inserted, the higher the charging capacity. Similarly, when the battery is discharged (that is, we the process of using the battery), the lithium ions embedded in the carbon layer of the negative electrode are released and move back to the positive electrode. The more lithium ions returned to the positive electrode, the higher the discharge capacity.
Generally, the charging current of a lithium battery is set between 0.2C and 1C. The larger the current, the faster the charging, and the greater the heat generated by the battery. Moreover, the capacity is not full enough for charging with excessive current,because the electrochemical reaction inside the battery takes time. Just like pouring beer, pouring too fast will produce foam, but dissatisfaction.
Precautions for use (discharge)
For batteries, normal use is the process of discharging. Points to note when discharging lithium batteries:
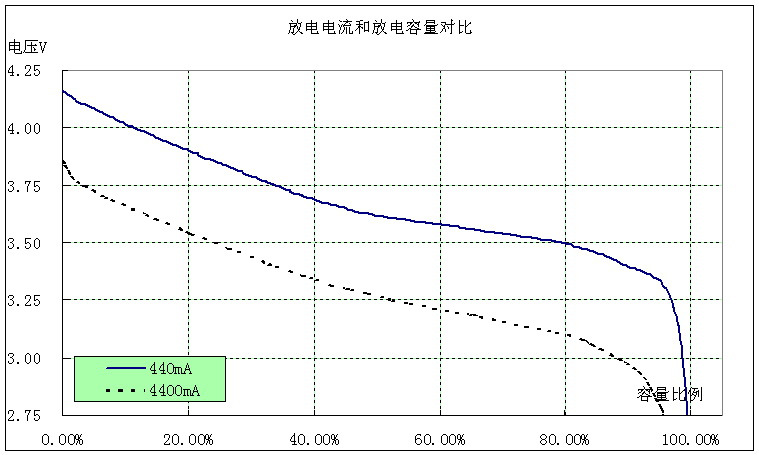
First, the discharge current should not be too large. Excessive current will cause heat inside the battery, which may cause permanent damage. On the phone, this is no problem, you can not consider.
It can be seen from Figure 1 that the larger the battery discharge current, the smaller the discharge capacity and the faster the voltage drop.
Second, it cannot be over-discharged. The internal storage of electric energy in lithium batteries is achieved by a reversible chemical change in electrochemistry. Excessive discharge will cause this chemical change to have an irreversible reaction.It should happen, so lithium batteries are most afraid of over-discharge. Once the discharge voltage is lower than 2.7V, the battery may be scrapped. Fortunately, a protection circuit has been installed inside the battery of the mobile phone, and the voltage It is not low enough to damage the battery, the protection circuit will work and stop discharging.
5. Lithium-ion battery production process
The positive electrode materials of lithium batteries include lithium cobalt oxide LiCoO2, ternary materials Ni+Mn+Co, lithium manganate LiMn2O4 plus conductive agent and binder, which are coated on aluminum foil to form the positive electrode, and the negative electrode is the layer Graphite plus conductive agent and adhesive are coated on the copper foil base tape, and the more advanced anode layered graphite particles have adopted nano-carbon.
1. Pulping: Use a special solvent and binder to mix with the powdered positive and negative active materials, and stir them evenly to make a slurry of positive and negative materials.
2. Coating: The positive and negative electrode slurry is uniformly coated on the surface of the metal foil by an automatic coating machine, and the positive and negative electrodes are automatically cut into positive and negative pole pieces after automatic drying.
3. Assembly: According to the top-down sequence of positive electrode sheet-diaphragm-negative electrode sheet-diaphragm, the process of injecting electrolyte, sealing, and welding the positive and negative poles to complete the battery Assembling process to make finished battery.
4. Formation: Put the finished battery in the test cabinet for charge and discharge test, screen out the qualified finished battery, and wait for it to leave the factory.
6. Li-ion battery safety features
Regarding the assessment indicators of the safety performance of lithium-ion batteries, very strict standards are stipulated in the world. A qualified lithium-ion battery should meet the following conditions in terms of safety performance:
(1) Short circuit: no fire, no explosion
(2) Overcharge: no fire, no explosion
(3) Hot box test: no fire, no explosion (constant temperature of 150℃ for 10min)
(4) Acupuncture: no explosion (use a Ф3mm nail to penetrate the battery)
(5) Plate impact: no fire, no explosion (10kg heavy object hits the battery from a height of 1M)
(6) Incineration: no explosion (gas flame grill battery)
7. The advantages of lithium ion batteries
1) High voltage
The working voltage of single battery is as high as 3.7-3.8V (3.2V for lithium iron phosphate), which is 3 times that of Ni-Cd and Ni-MH batteries.
2) Greater than energy
The actual specific energy that can be achieved is about 555Wh/kg, that is, the material can reach a specific capacity above 150mAh/g (3-4 times Ni-Cd, 2-3 times Ni-MH), which is close to its theory.About 88% of the value.
3) Long cycle life
Generally, it can reach more than 500 times, or even more than 1000 times, and it can reach 8000 times for lithium iron phosphate. For electrical appliances with low current discharge, the battery life will be doubled by the electrical appliances Competitiveness.
4) Good safety performance
No pollution, no memory effect. As the predecessor of Li-ion, the lithium battery is short-circuited due to the easy formation of dendrites due to metal lithium, which reduces its application fields: Li-ion does not contain cadmium, lead, or mercury and other elements that pollute the environment; a major disadvantage of Ni-Cd batteries of some processes (such as sintered) is the "memory effect", which severely restricts the use of batteries, but Li-ion there is no such problem at all.
5) Small self-discharge
The self-discharge rate of fully charged Li-ion stored at room temperature for 1 month is about 2%, which is much lower than 25-30% of Ni-Cd and 30-35% of Ni-MH.
6) Fast charging
The capacity of 1C charging in 30 minutes can reach more than 80% of the nominal capacity, and the ferro-phosphorus battery can be charged to 90% of the nominal capacity in 10 minutes.
7) Working temperature
The working temperature is -25~45°C. With the improvement of electrolyte and positive electrode, it is expected to expand to -40~70°C.
8. Disadvantages of lithium-ion batteries
senescence
Unlike other rechargeable batteries, the capacity of lithium-ion batteries will slowly decline, which is related to the number of uses and temperature. This phenomenon of decline can be represented by a decrease in capacity,
It can also be expressed as an increase in internal resistance.
Because it is related to temperature, it is easier to reflect in electronic products with high working current. Replacing graphite with lithium titanate seems to extend life. Permanent loss of storage temperature and capacity
| Charging power | Storage temperature0℃ | Storage temperature25℃ | Storage temperature40℃ | Storage temperature60℃ |
| 40%~60% | 2%/year | 4%/year | 15%/year | 25%/year |
| 100% | 6%/month | 20%/month | 35%/month | 80%/6month |
Speed relationship:
Recovery rate
Approximately 1% of new products leaving the factory need to be recycled for various reasons.
Intolerant of overcharging
When overcharged, the excessively inserted lithium ions will be permanently fixed in the crystal lattice and can no longer be released, which can result in a short battery life.
Intolerance of overdischarge
During over-discharge, the electrode deintercalates too much lithium ions, which can cause the crystal lattice to collapse and shorten the life.
 sales@batterydeji.com
sales@batterydeji.com




